Road to HTA Institutionalization: Where We Are and Where Are We Headed?
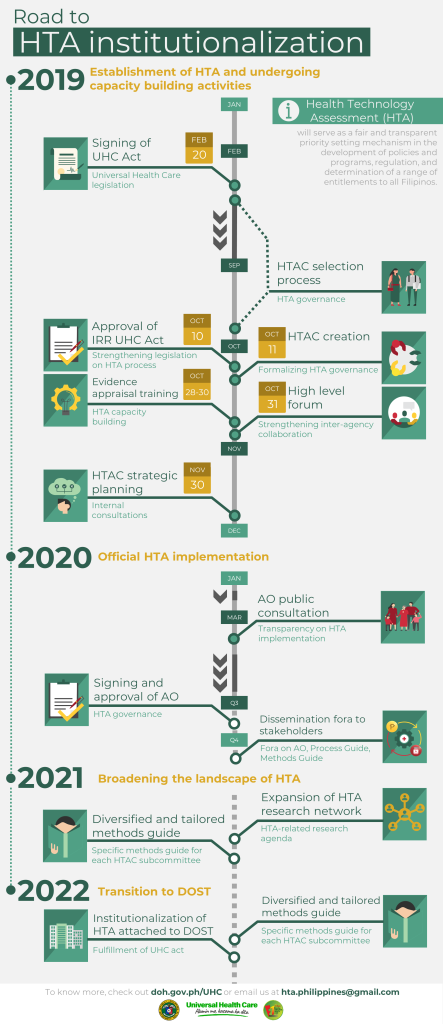
Ensuring access to effective, efficient, affordable and quality healthcare for every Filipino is one of the goals of Republic Act 11223 otherwise known as the “Universal Health Care (UHC) Act.” In the collective vision to progressively realize UHC, different health interventions and technologies would need to undergo a systematic process called Health Technology Assessment (HTA) to serve as a fair and transparent priority setting mechanism in the development of policies and programs, regulation, and determination of a range of entitlements to all Filipinos.
To ensure its effective implementation, HTA is governed by the Health Technology Assessment Council (HTAC), which is composed of nine core committee members led by Dr. Marita Tolentino-Reyes, who is an ethics practitioner.
Subcommittees for (1) drugs, (2) vaccines, (3) clinical equipment and devices, (4) medical and surgical procedures, (5) preventive and promotive health services, (6) traditional medicine; (7) other health technologies – with three members each – are created to assist the core committee. The DOH-HTA unit is also established under the Health Regulation Team to provide evidence generation and secretariat support.
The Council’s mandate is to facilitate the provision of financing and coverage recommendations on health technologies financed by DOH and PhilHealth, oversee and coordinate the HTA process, and review and assess existing DOH and PhilHealth benefit packages.
Many work streams have been initiated in 2020 to officially lay down the implementation of HTA, particularly the signing and issuance of the HTA Administrative Order (AO) and the HTA Process and Methods Guide. These documents were peer-reviewed by international experts and went under the scrutiny of HTAC and various stakeholders from different sectors through an online public consultation via website posting.
Currently, the AO, and the HTA Process and Methods Guide are under finalization. Completion of this work stream is targeted by the end of the 3rd quarter of 2020. This will then be followed by a series of webinars in the 4th quarter of 2020, which aim to introduce the provisions of the AO and familiarize the stakeholders with the official HTA process and methods.
Aside from the officialization of the HTA’s process and general methods, the DOH-HTA unit has also partnered with established international HTA agencies- UK National Institute for Health and Care Excellence (NICE) and Thailand’s Health Intervention and Technology Assessment Program (HITAP). These partnerships shall serve as a guide in building a local HTA institution which meets the standards of the international community. A number of capacity building activities with international partners has been organized since 2019 and is being continuously planned to ensure the quality of the process and methods employed in the conduct of HTA in our country.
In the year 2021, efforts will be focused on broadening the landscape of HTA in the Philippines. The general methods guide shall be diversified and tailored depending on the specific methodological needs of different health technologies. A more specific methods guide for clinical equipment and device, medical and surgical procedures, and traditional medicine shall be developed. Alongside this, the HTA research network shall be expanded in coordination with the Department of Science and Technology (DOST) to facilitate the implementation of all research agenda related to HTA.
In its third year of establishment in 2022, it is the goal to fully institutionalize HTAC at DOST as an independent recommendatory body to DOH and PhilHealth. This is in fulfillment of the UHC law which mandates HTAC to be an independent entity separate from DOH as an attached agency to DOST within five years upon its establishment. The establishment of specific HTA methodological guidelines for preventive and promotive health services, and other health technologies shall also be developed.
