The HTA Council hereby makes public the 2024 Provisional List of Priority HTA Topics for stakeholder consultation from 12 to 26 April 2024. Only appeals that are based on the acceptable grounds, have complete sets of requirements, and submitted on time to the correct email address will be processed. Full list of topics and important continue reading : [Advisory] Release of the Provisional TP List (April 2024)
HTA Council Recommendation on Bivalent COVID-19 Vaccines as Booster
HTA Council Recommendation on Bivalent COVID-19 Vaccines as booster Date of OIC-SOH Approval: 25 April 2023 This assessment followed the HTA Council evaluation framework to evaluate COVID-19 vaccines using the following criteria: (1) responsiveness to magnitude and severity; (2) clinical efficacy and safety; (3) affordability, viability and feasibility; (4) household financial impact; (5) social impact; and continue reading : HTA Council Recommendation on Bivalent COVID-19 Vaccines as Booster
[Announcement] HTA Council Releases Its Preliminary Recommendation on the Financing of DOACs for Nonvalvular Atrial Fibrillation
The Health Technology Assessment (HTA) Council releases its preliminary recommendation on the government financing of apixaban, dabigatran, and rivaroxaban for the prevention of myocardial infarction, cerebrovascular diseases and other cardiovascular events among patients with nonvalvular atrial fibrillation (NVAF) and subgroups of NVAF patients through its inclusion in the Philippine National Formulary.Appeals will be accepted from continue reading : [Announcement] HTA Council Releases Its Preliminary Recommendation on the Financing of DOACs for Nonvalvular Atrial Fibrillation
[Announcement] HTA Council Releases Its Preliminary Recommendation on the Non-Financing of Cerebrolysin for Post Ischemic Stroke
The Health Technology Assessment (HTA) Council releases its preliminary recommendation on the non-government financing of cerebrolysin in combination with rehabilitation or standard of care for the treatment of adults post-ischemic stroke through its non-inclusion in the Philippine National Formulary.Appeals will be accepted from 17 March 2023 to 31 March 2023 Kindly see full advisory:CALL FOR continue reading : [Announcement] HTA Council Releases Its Preliminary Recommendation on the Non-Financing of Cerebrolysin for Post Ischemic Stroke
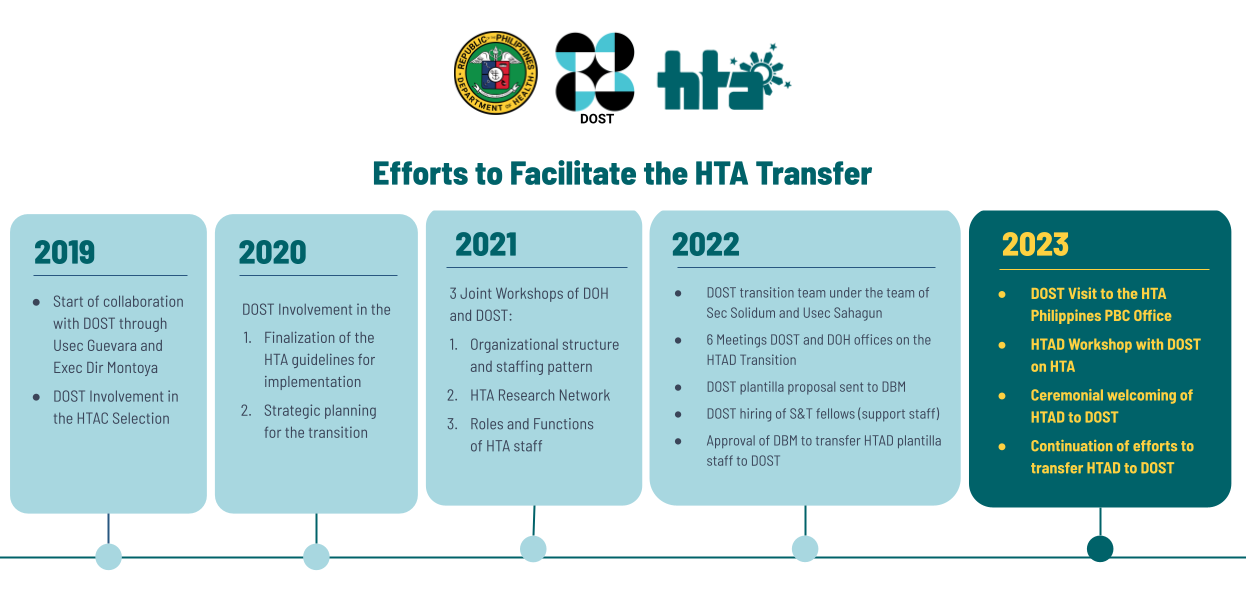
HTA Philippines Guidance: Efforts to Facilitate the HTA Transfer
The Department of Health (DOH) officially transferred the operations of the Health Technology Assessment (HTA) Philippines to the Department of Science and Technology (DOST) through a ceremonial turnover of the HTA Council and HTA Division from the DOH to the DOST on 06 March 2023 at the Philippine International Convention Center (PICC), Manila. To facilitate continue reading : HTA Philippines Guidance: Efforts to Facilitate the HTA Transfer

