Issuances
2024 Issuances
-
HTA Council Recommendation on Calcium Hydroxide 80% + Clinoptilolite 20% (Kiti-kitiX) Larvicide
September 10, 2024
-
[Advisory] Release of HTA Topic Prioritization List 2024 – Cycle 2 Topics that will proceed to Topic Assessment
May 24, 2024
-
EXTENSION OF THE STAKEHOLDER APPEALS PERIOD FOR THE 2024 PROVISIONAL LIST OF PRIORITY HTA TOPICS
April 24, 2024
-
[Advisory] Release of the Provisional TP List (April 2024)
April 17, 2024
2023 Issuances
-
[Announcement] HTA Council Releases Its Preliminary Recommendation on the Financing of DOACs for Nonvalvular Atrial Fibrillation
March 24, 2023
-
[Announcement] HTA Council Releases Its Preliminary Recommendation on the Non-Financing of Cerebrolysin for Post Ischemic Stroke
March 24, 2023
-
HTAC Guidance: PNF Inclusion of Recombinant Human Erythropoietin (Epoetin Alfa) 4000 IU/mL Solution for Injection (IV/SC) 1mL Pre-filled Syringe
March 14, 2023
-
HTAC Guidance: PNF Inclusion of Filgrastim 300 mcg/0.9 mL Solution (IV, SC), 0.9 mL Pre-filled Syringe
March 14, 2023
-
[Announcement] Ceremonial Turnover of HTA Implementation from the Department of Health to the Department of Science and Technology
March 9, 2023
-
[Announcement] HTA Council Releases Its Preliminary Recommendation on the Financing of Dapagliflozin for Patients with Type 2 Diabetes Mellitus
March 8, 2023
-
[Department Circular No. 2023-0059] Health Technology Assessment Council (HTAC) Recommendation for the Inclusion of Deferasirox 90 mg and 180 mg Film-Coated Tablet (FCT) for Chronic Iron Overload due to Repetitive Blood Transfusions in Patients 2 Years Old and Above in the Philippine National Formulary (PNF)
February 13, 2023
-
[Department Circular No. 2023-0061] Health Technology Assessment Council (HTAC) Recommendation for the Inclusion of Filgrastim 300 mcg/0.9 mL Solution (IV, SC), 0.9 mL Pre-filled Syringe for the Treatment of Neutropenia in Patients Undergoing Bone Marrow Transplantation and Patients Receiving Myelosuppressive Cancer Chemotherapy in the Philippine National Formulary (PNF)
February 13, 2023
-
[DC No. 2023-0060] Health Technology Assessment Council (HTAC) Recommendation for the Inclusion of Recombinant Human Erythropoietin (Epoetin Alfa) 4000 IU/mL Solution for Injection (IV/SC) 1mL Pre-filled Syringe for the Management of Chemotherapy-Induced Anemia in Patients with Non-Myeloid Disease in the Philippine National Formulary (PNF)
February 13, 2023
2022 Issuances
-
[Advisory] Extension of the Call for Nominations to the Health Technology Assessment Council (HTAC) Core Committee and Subcommittee Members until 27 January 2023
December 22, 2022
-
[Department Circular No. 2022 – 0625] Health Technology Assessment Council (HTAC) Recommendation for the Inclusion of Abiraterone Acetate and Non-Inclusion of Enzalutamide for Individuals with Metastatic Castration-Resistant Prostate Cancer in the Philippine National Formulary (PNF)
December 15, 2022
-
[Department Memorandum No. 2022 – 0545] Interim Guidelines on the Process and Methods for the Selection of Medical Devices for Inclusion in the Philippine Essential Medical Device List (PEMDL)
December 14, 2022
-
[Advisory] Publication of the Guidelines on the Application of the Philippine Social Values on the Health Technology Assessment (HTA)
December 13, 2022
-
[Advisory] Extension of the Call for Nominations to the Health Technology Assessment Council (HTAC) Core Committee and Subcommittee Members until 07 December 2022
November 14, 2022
-
[Department Personnel Order No. 2022 – 3735] Appointment of the Health Technology Assessment Council Core and Subcommittees
November 10, 2022
-
[Department Circular No. 2022 – 0584] Amendment to the Department Circular 2022-0254 dated 05 April 2022 titled, “Updates on the HTAC Processing of Minor Inclusion”
November 9, 2022
-
[Department Circular No. 2022 – 0580] Release of the Final Priority List of HTA Topics for 2022 That Will Proceed to Topic Assessment
November 7, 2022
-
[Department Circular No. 2022 – 0560] Health Technology Assessment Council (HTAC) Recommendation for the Inclusion of Zoledronic Acid for Individuals with Malignancy-Related Bone Disease in the Philippine National Formulary (PNF)
October 28, 2022
-
[Department Personnel Order No. 2022 – 3725] Appointment of the Health Technology Assessment Council Core and Subcommittees
October 20, 2022
-
[Department Circular No. 2022 – 0537] Health Technology Assessment Council (HTAC) Recommendation for the Inclusion of Insulin Glargine and Non inclusion of Insulin Detemir for Patients with Type 1 or Type 2 Diabetes Mellitus in the Philippine National Formulary (PNF)
October 14, 2022
-
HTAC Preliminary Recommendation on Abiraterone Acetate (250mg Tablet) and Enzalutamide (40mg Soft Gel Capsule)
October 4, 2022
-
[Department Circular No. 2022 – 0291] Amendment to the Department Circular 2022-0291 “Health Technology Assessment Council (HTAC) Recommendation to include Potassium Citrate 15 mEq Extended Release (ER) Tablet in the Philippine National Formulary”
September 27, 2022
-
[Department Circular No. 2022 – 0491] Nomination to the Health Technology Assessment Council (HTAC) Core Committee and Subcommittee Members
September 23, 2022
-
[Department Circular No. 2022 – 0486] Health Technology Assessment Council (HTAC) Recommendation for the Non-inclusion of Eribulin as Second-line Treatment of Metastatic Soft Tissue sarcoma (mSTS) the Philippine National Formulary (PNF)
September 20, 2022
-
[Advisory] Draft Department Memorandum on the Methodology of PEMDL Draft Interim Guidelines on the Process and Methods for the Selection of Medical Devices for Inclusion in the Philippine Essential Medical Device List (PEMDL) for Comments until 21 September 2022
September 14, 2022
-
HTAC Preliminary Recommendation for Government Financing of Zoledronic Acid for Individuals with Malignancy-Related Bone Disease
September 7, 2022
-
[Department Memorandum No. 2022- 0379] Updated Checklist of Requirements for the Health Technology Assessment of COVID-19 Health Technologies to be referred by the Disease Prevention and Control Bureau (DPCB) and National Vaccine Operations Center (NVOC)
September 2, 2022
-
[Department Circular No. 2022- 0400] Change in the Generic Name of “Nicotine Polacrilex Medicated Chewing Gum” in the Philippine National Formulary (PNF) to “Nicotine”
August 4, 2022
-
Preliminary Recommendation for the Inclusion of Insulin Glargine and Insulin Detemir for Type 1 and Type 2 Diabetes Mellitus in the PNF
July 26, 2022
-
Preliminary Recommendation for the Non-Inclusion of Eribulin in the Treatment of Soft Tissue Sarcoma in the PNF
June 15, 2022
-
[Department Personnel No. 2022- 2048] Creation of a Screening Committee for the Appointment of Health Technology Assessment Committee (HTAC) Members
June 13, 2022
-
[Department Circular No. 2022- 0288] Nomination to the Health Technology Assessment Council (HTAC) Social Anthropologist Core Committee Member
June 10, 2022
-
[Department Circular No. 2022- 0291] Health Technology Assessment Council (HTAC) Recommendation to include Potassium Citrate 15 mEq Extended Release (ER) Tablet in the Philippine National Formulary
June 6, 2022
-
[Department Circular No. 2022- 0289] Health Technology Assessment Council (HTAC) Recommendation to include Tocilizumab in the Philippine National Formulary
June 1, 2022
-
[Department Circular No. 2022- 0257] Changes to the Topic Nomination and Appeals Processes of the Philippine HTA Process Guide
May 27, 2022
-
[Department Circular No. 2022- 0254] Updates on the HTAC processing of Minor Inclusion Application
May 20, 2022
-
[Department Personnel No. 2022- 2780 – A] Amendment to the Department Personnel Order No. 2021-2780 Entitled “Creation of the Pool of Clinical Experts for Consultation on the Evaluation of Assessment Topics” to Include Expert Advisory Committee as Additional Experts
May 17, 2022
-
[Department Personnel Order No. 2022- 1245] Appointment of the New Member of the Health Technology Assessment Council Subcommittee on Drugs
May 10, 2022
-
Preliminary Recommendation for the Inclusion of Potassium Citrate 1620 mg (15 mEq) Tablet in the PNF
April 13, 2022
-
[Department Personnel Order No. 2022- 1102] Authority for Select Department of Health (DOH) Personnel to conduct a Hybrid Workshop on the Development of Guidelines in the Creation of the Philippine Essential Medical Device List (PEMDL) on April 22, 2022 in Metro Manila,
April 7, 2022
-
[Department Circular No. 2022- 0183] Opening of the 2022 Call for topic nomination
April 1, 2022
-
[Department Circular No. 2022- 0164] Health Technology Assessment Council (HTAC) Recommendation for reinclusion of vasopressin 20 iU/ml (IM/IV) in the Philippine National Formulary
March 23, 2022
-
Preliminary Recommendation for the Inclusion of Rituximab 1400 mg/11.7 mL Solution for Subcutaneous (SC) Injection for the Treatment of Non-Hodgkin’s Lymphoma
March 4, 2022
-
Preliminary Recommendation for the Inclusion of Vasopressin 20 IU/mL (IM/IV)
February 11, 2022
-
[Department Circular No. 2022- 0074] Nomination to the Health Technology Assessment Council (HTAC) Subcommittee on Drugs
February 9, 2022
-
[Department Circular No. 2022- 0015] Health Technology Assessment Council (HTAC) Recommendation to Include Sambong 250mg Tablet in the Philippine National Formulary
January 21, 2022
-
Preliminary Recommendation for the Non-Inclusion of Pertuzumab for Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer
January 20, 2022
-
[Department Personnel Order No. 2021-3184] Appointment of the New Member of the Health Technology Assessment Council Subcommittee on Preventive and Promotive Health
January 5, 2022
2021 Issuances
-
Preliminary Recommendation for the Inclusion of Sambong 250mg Tablets as Anti-Urolithiasis
December 23, 2021
-
Preliminary Recommendation on Emtricitabine + Tenofovir Disoproxil Fumarate fixed-dose combination as Oral Pre-Exposure Prophylaxis (PrEP)
December 16, 2021
-
[Department Personnel Order No. 2021-2780] Creation of Pool of Clinical Experts for Consultation on the Evaluation of Assessment Topics
December 10, 2021
-
[Department Circular No. 2021- 0471] Nomination to the Health Technology Assessment Council (HTAC) Subcommittee on Preventive and Promotive Health
October 22, 2021
-
[Department Circular No. 2021- 0468] Health Technology Assessment Council (HTAC) Recommendations on COVID-19 Vaccination of the Pediatric Population
October 22, 2021
-
[Signed by Secretary of Health] HTAC Recommendations on Booster and Additional Dose
October 21, 2021
-
[Signed by Secretary of Health] HTAC Interim Recommendations on Pediatric Vaccination for 2022 COVID-19 Vaccine Implementation
October 15, 2021
-
[Signed by Secretary of Health] September Updates on 2021 HTAC Recommendations for Rapid Antigen Tests
October 12, 2021
-
[Department Circular No. 2021- 0423] Inclusion of Dolutegravir (DTG) in the Philippine National Formulary (PNF)
September 28, 2021
-
[Signed by Secretary of Health] Letter of Endorsement for Dolutegravir for the Second-line Treatment for Treatment Experienced Adolescents and Adults Living with HIV
September 21, 2021
-
[Department Circular No. 2021- 0410] Inclusion of Two-Dose Inactivated Polio Vaccine (IPV) in the Philippine National Formulary (PNF)
September 20, 2021
-
[Department Circular No. 2021- 0400] Inclusion of Tenofovir/Lamivudine/Dolutegravir (TLD) in the Philippine National Formulary (PNF)
September 10, 2021
-
[Department Circular No. 2021- 0376] Interim Requirements for the Health technology Assessment (HTA) of Medical Devices
September 3, 2021
-
[Department Memorandum No. 2021- 0367] Health Technology Assessment Council’s (HTAC) Updated Recommendation on COVID-19 Vaccine Sinovac
August 27, 2021
-
Preliminary Recommendation on Dolutegravir (DTG)
August 27, 2021
-
[Department Circular No. 2021- 0337] Rescheduling of the Opening HTA Topic Nominations to March 2022
August 17, 2021
-
[Department Circular No. 2021- 0331] The Health Technology Assessment Council (HTAC) Releases Its Updated Evidence Summaries for COVID-19 Vaccine Assessments
August 17, 2021
-
MEMORANDUM- Request for Comments on Evaluation Framework for COVID-19 Vaccines (Version 2)
August 16, 2021
-
[Department Circular No. 2021- 0348] Coresia Vaccine Certificate Survey for Key Stakeholders
August 10, 2021
-
Preliminary Recommendation on Tenofovir/Lamivudine/Dolutegravir (TLD)
August 9, 2021
-
[Department Memorandum No. 2021- 0317] Non-inclusion of Pazopanib as a Second-line Treatment for Metastatic Soft Tissue (mSTS) Sarcoma in the Philippine National Formulary (PNF)
July 21, 2021
-
[Department Circular No. 2021- 0273] HTAC Issuance on Acceptance and processing of Health Technologies under Monitored Release with Phase IV Trial Data
July 20, 2021
-
Preliminary Recommendation on Two-Dose Inactivated Polio Vaccine (IPV)
July 13, 2021
-
[Department Memorandum No. 2021- 0294] Non-inclusion of Lapatinib in the Philippine National Formulary
July 5, 2021
-
[Department Memorandum No. 2021- 0264] Health Technology Assessment Council’s (HTAC) Recommendation on COVID-19 Vaccine Moderna
June 9, 2021
-
[Department Memorandum No. 2021- 0252] Health Technology Assessment Council’s (HTAC) Recommendations on RT-PCR and Rapid Antigen Testing for COVID-19
June 1, 2021
-
Preliminary Recommendation on Lapatinib
May 31, 2021
-
[Department Memorandum No. 2021- 0242] Inclusion of Nicotine Replacement Therapy (NRT) and Varenicline in the Primary Care Formulary
May 27, 2021
-
[Department Memorandum No. 2021- 0227] Health Technology Assessment Council’s (HTAC) Recommendations on COVID-19 Health Technologies
May 17, 2021
-
[Department Memorandum No. 2021- 0199] Health Technology Assessment Council’s (HTAC) Recommendations on COVID-19 Health Technologies
April 23, 2021
-
[Department Memorandum No. 2021- 0158] Inclusion of 0.1% Sodium Hyaluronate (15 ml) and 2.5% Peritoneal Dialysis Solution (2L and 5L) in the Philippine National Formulary
April 5, 2021
-
[Department Circular No. 2021- 0079] Health Technology Assessment Council’s (HTAC) Recommendations on health technologies requiring Full Certificate of Registration (CPR) from the Food and Drug Administration
March 5, 2021
-
[Department Memorandum No. 2021- 0075] Compliance to Dissemination of the Recommendations of the Health Technology Assessment Council (HTAC)
February 26, 2021
-
[Department Memorandum No. 2021- 0097] Health Technology Assessment Council’s (HTAC) Recommendations on COVID-19 Health Technologies
February 26, 2021
-
[Memo to DOH Internal Offices] Acknowledgement of Comments_Concurrence on COVID-19 Vaccines Evaluation Framework
February 15, 2021
-
[Department Memorandum No. 2021- 0079] Endorsement of Topic Submissions from National Health Programs for HTA
February 10, 2021
-
[Department Memorandum No. 2021- 0079] Endorsement of Topic Submissions from National Health Programs for Health Technology Assessment
February 10, 2021
-
[DOH Department Circular] Submission of Data of COVID-19 Vaccines under EUA
February 2, 2021
2020 Issuances
-
[Memo to DOH Internal Offices] Consultation for COVID-19 Vaccines Evaluation Framework.
December 28, 2020
-
Memo on the Acceptance of Minor Inclusions in the Philippine National Formulary (PNF) for Health Technology Assessment (HTA) Following the PNF System for Drugs
November 18, 2020
-
Extension of Topic Nomination (Existing Health Technologies) for Health Technology Assessment until 10 November 2020
September 21, 2020
-
[Department Memorandum No. 2020-0366] Inclusion of 10- and 13-valent Pneumococcal Conjugate Vaccines (PCV) Multidose Preparation in the Philippine National Formulary
August 14, 2020
-
[Advisory] Consultative Meeting for Administrative Order 2020-0041
March 11, 2020
-
[Department Circular No. 2020-0011] Non-Acceptance of Requests for Exemption from the Philippine National Formulary
January 23, 2020
Latest Assessments
Ongoing Development
Events and Press Releases

We are pleased to invite you to join the Meeting with Stakeholders for the 2024 Provisional HTA Topic Prioritization List – Cycle 2 Topics on 23 April 2024 (Tuesday) at 1:00-3:00 PM via Zoom teleconference. Kindly see the advisory for full details: bit.ly/SHMeetingProviList ————————————————————————– To keep updated on HTA news, visit our website: https://hta.dost.gov.ph/ Like and...
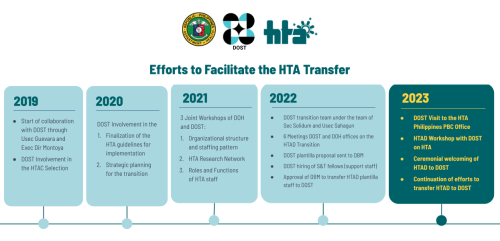
The Department of Health (DOH) officially transferred the operations of the Health Technology Assessment (HTA) Philippines to the Department of Science and Technology (DOST) through a ceremonial turnover of the HTA Council and HTA Division from the DOH to the DOST on 06 March 2023 at the Philippine International Convention Center (PICC), Manila. To facilitate...

The Department of Health (DOH) officially transferred the operations of the Health Technology Assessment (HTA) Philippines to the Department of Science and Technology (DOST) through a ceremonial turnover of the HTA Council and HTA Division from the DOH to the DOST on 06 March 2023 at the Philippine International Convention Center (PICC), Manila. DOH Officer-in-Charge-Secretary...










